Figure 1
You are using a web browser that is not fully supported by this website. Some features may not work as intended. For the best experience, please use one of the recommended browsers.

Opening Image: Diamond. PDB file courtesy of Georgia Tech.
Diamond is a pure carbon mineral. Each carbon is in sp3 orbital configuration and is bonded to four other carbons with similar electronic configurations. the formation of the crystal lattice. This tetrahedral arrangement of covalent bonds in diamond accounts for its extraordinary hardness and high melting point (3500°C).
This series on noncovalent interactions deliberately avoids any detailed description and covers those aspects and concepts required for dealing with structural biology at the molecular scale.
Let's deal first with the term, noncovalent interactions. You are probably familiar with the term intermolecular forces from previous courses. However, in dealing with proteins and nucleic acids, we are interested in intramolecular noncovalent interactions which stabilize or destabilize these macromolecules, as well as intermolecular interactions which contribute to the stability of noncovalent complexes between molecules in the biological world. However, the types of interactions are the same in both cases and include charge-charge, ion-dipole, dipole-dipole, and van der Waals interactions. Click here for a table.
The only interactions possible between nonpolar molecules are the van der Waals forces. The helium atom has a closed electron structure and no chemical bonding may occur. Were it not for the weak short-range attractive forces -- the van der Waals forces -- gaseous helium could not be condensed into a liquid or a solid phase. As it is, the force of attraction between two helium atoms (0.076 kJ/mol) is so weak that at a temperature of only 4.2 K they have sufficient kinetic energy to overcome the forces of attraction between them and escape into the gas phase.
Van der Waals attractions work because, at close range, the helium atoms notice each other's dipole moments. The dipole moments are due to the fact that at any given instant, the electron cloud is not quite centered on the nucleus of the atom (although it will be centered on average). This instantaneous dipole moment causes atoms in the vicinity to arrange their instantaneous dipoles so as to lower their energy, which causes attraction.
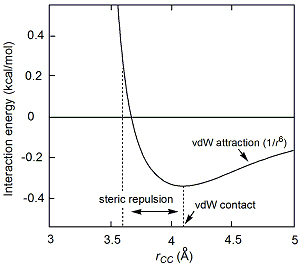
The van der Waals interaction energy can be calculated by applying quantum mechanics. The result for a pair of methane molecules in the gas phase are shown in .
Even though CH4 has no net dipole, at any instant its electron density may not be completely symmetrical, resulting in an instantaneous dipole. This can induce an instantaneous dipole in another methane molecule. However, because methane has more nuclei and more electrons than helium the van der Waals interaction energy for two methane molecules is -1.5 kJ/mol (-0.36 kcal/mol x 4.18 kJ/kcal), compared to -0.076 kJ/mol for helium. Helium boils at 4.2 K, while methane boils at 112 K.
Pentane isomers. Van der Waals interaction energies are largely dictated by the surface area of contact and are relatively insensitive to the precise identities of the groups involved. The isomers of pentane differ in their overall shape. Being nonpolar, they are held together only by the weak van der Waals forces, which are effective only when the molecules are in contact. Thus, between isomers, the more surface area a molecule has, the greater the van der Waals forces holding the molecules together and the higher the boiling point. Of the C5H12 isomers, pentane is the most extended and flips back and forth between conformations. Because the different conformations can intermingle with each other, pentane has the highest boiling point at 36.1°C. -- (CH3)4C -- is almost spherical and the contact surface between neopentane molecules is smaller, so neopentane has the lowest boiling point at 9.5°C.
Graphite. The distance between layers is 3.34 Å, the same as that between base pairs in B DNA. spacefill.
Although van der Waals interactions are weak on a per-atom basis, the sum of van der Waals interactions between smooth surfaces can easily be observed at the macroscopic level.
Graphite is an excellent example of van der Waals forces at the macroscopic level. Like diamond, graphite is composed solely of carbon. Diamond's hardness is due to the fact that all the carbon atoms in the crystal are covalently bonded to each by tetrahedral sp3 orbitals. Graphite, however, is soft and you can easily crush it with your fingers. In graphite, the carbon atoms are arranged in sheets. Each carbon atom is surrounded by three other carbon atoms with 120° bond angles due to the sp2 orbitals of the carbon atoms. The overlap to form π orbitals, thus locking the carbon atoms in a smooth flat surface for each individual sheet in the crystal. The sheets are held together by van der Waals forces. These relatively weak interactions allow the sheets to easily slide past each other, providing the greasy feel common to graphite, as well as its lubricating properties.
Talc is an extremely soft mineral used in baby powder (talcum powder). It's a magnesium silicate mineral made of sheets. As in graphite, these electrostatically neutral layers are held together by van der Waals interactions, giving rise to its soft and slippery nature.
Silicon and magnesium are shown in CPK colors; beige and green, respectively. Note the octahedral coordination to the Mg2+ ions in the center of each layer. The van der Waals surfaces of graphite and talc are hydrophobic. spacefilling.
How do we define contacts between two protein molecules? We simply ask the computer to search for atoms in one molecule or subunit which are close enough to maintain van der Waals interactions with atoms in the adjacent subunit. Since van der Waals or dispersion forces are very short range, the cutoff distance for significant interaction between atoms is the sum of the van der Waals radii plus 0.5 Å. That means any visible spaces between the contact surfaces of the dimer are less than 0.5 Å.
We can show the regions which comprise contact surfaces by using different colors in the model. To show you how this looks, let's examine human defensin HNP-3, one of the smallest dimers in the Protein Data Bank. The monomer has 30 amino acid residues and folds into a unique tertiary structure. When the monomers meet, they associate to form a dimer. The interactions that stabilize the dimer include van der Waals forces at the region where the two subunits fit together. Click here to learn more about defensins.
Crystal structure of human defensin HNP-3. The molecule consists of two identical chains: A and B. The oxygen atoms of the ordered water molecules are shown in dark blue to distinguish them from the oxygen atoms of the peptide. Most of the ordered water molecules are hydrogen bonded to peptide carbonyl oxygen atoms or amide –NH groups.
of chain A. Atoms in van der Waals contact with atoms of chain B are shown in a lighter shade. Notice the absence of water molecules at the interface. of chain B.
of the dimer. The contact residues at the interface are shown in lighter shades. to isolate the interface. Remember that the cutoff distance for significant van der Waals interaction between atoms is the sum of the van der Waals radii plus 0.5 Å. That means any visible spaces between the contact surfaces of the dimer are less than 0.5 Å. As you can see, the two surfaces fit together fairly closely. In other words, the defensin monomers meet one criterion for binding to each other, namely, shape complementarity -- the most basic ingredient of molecular recognition. We'll explore other criterion in later lectures.
Although very weak on a per-atom basis, van der Waals interactions play an important role in the stability of biological macromolecules and molecular assemblies. Van der Waals interaction energies are largely dictated by the surface area of contact and are relatively insensitive to the precise identities of the groups involved. The greater the steric complementarity at the interface between two macromolecules in a complex, the closer the atoms and the greater the sum of van der Waals attractions.
Although the hydrophobic effect accounts for the stability of the defensin dimer, the reason that the exposed hydrophobic "patch" of one defensin monomer associates specifically with the hydrophobic patch of another defensin molecule -- and not with exposed hydrophobic regions of other molecules -- depends in part on van der Waals interactions arising from the steric fit between the contact surfaces.