| Noncovalent Interaction | Interacting Species | Dependence of Energy on Distance | Strength | Directional |
| charge-charge (also called ion-ion or coulombic) |
charges or ions only; interaction between like charges is destabilizing (positive) | 1/r | strong | no |
| ion-dipole | ions and polar molecules | 1/r2 | moderate | yes |
| dipole-dipole | polar molecules | 1/r3 | weak | yes |
| London dispersion; van der Waals (attractive) |
nonpolar molecules | 1/r6 | very weak | no |
| hydrogen bond | O–H•••A or N–H•••A, where A = N or O | length is fixed | weak | highly directional |
Here are the details:
- Dipoles come in three flavors: permanent, induced, and instantaneous.
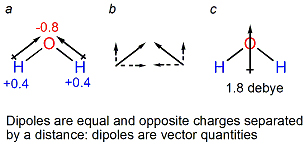
- Permanent dipole. Dipoles are equal and opposite charges q separated by a distance l. A permanent dipole occurs when a charge separation is always present in a molecule. Dipoles are oriented within a molecule, indicated by a vector l pointing from +q to –q. A permanent dipole is characterized by its dipole moment μ = ql. Dipole moments are measured in units of debyes (D). For example, water, which has no net charge, has a permanent dipole moment of 1.85 D, due to the partial negative charge on the oxygen and the partial negative charges on the two hydrogens:
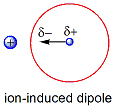
- Induced dipole. An induced dipole is a charge separation that arises only in the presence a charge or ion.
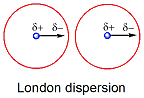
- Instantaneous dipole. The London or van der Waals force, which is generally attractive in nature, is a short range force and decays rapidly to zero away from a surface. The origin of the van der Waals force lies in the instantaneous dipole generated by quantum mechanical fluctuations in the electron cloud surrounding the nucleus of electrically neutral atoms. This leaves a partial positive charge on the nucleus and a region of excess electron density in the electron shell of the atom. This instantaneous dipole in turn induces a dipole in a nearby atom. The disturbance travels at the speed of light. Since the theoretical treatment is similar to that for dispersion of light waves, we sometimes refer to van der Waals forces as dispersion forces or London dispersion forces, after an American physicist who developed the theory. Since the electron cloud around a nonpolar molecule responds almost instantaneously to the presence of the first dipole, "induced dipole-induced dipole" interactions don't depend on orientation.
- Permanent dipole. Dipoles are equal and opposite charges q separated by a distance l. A permanent dipole occurs when a charge separation is always present in a molecule. Dipoles are oriented within a molecule, indicated by a vector l pointing from +q to –q. A permanent dipole is characterized by its dipole moment μ = ql. Dipole moments are measured in units of debyes (D). For example, water, which has no net charge, has a permanent dipole moment of 1.85 D, due to the partial negative charge on the oxygen and the partial negative charges on the two hydrogens:
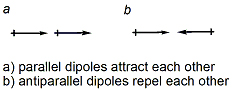
- Noncovalent interactions can be explained as electrostatic interactions. The strongest intra- or intermolecular interactions occur between two charged groups or ions. Electrostatic (coulombic) forces are proportional to charge. Therefore, charge-charge (ion-ion) interactions are the strongest and dipole-dipole interactions the weakest. Charge-charge interactions are long-ranged (1/r), whether stabilizing or destabilizing. Interactions become weaker and act at shorter distances as dipoles become involved. As you might expect, ion-dipole and dipole-dipole interactions depend on the orientations of the dipole. The two extremes for dipole-dipole interactions are: