Cooperative Binding of Thiostrepton
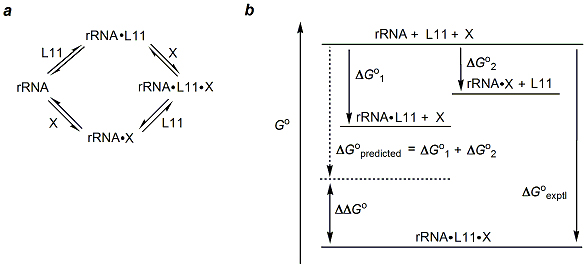 The binding of L11 and thiostrepton to the rRNA fragment 1051-1109 are linked in a thermodynamic cycle such that the binding of either one facilitates the binding of the other.
The binding of L11 and thiostrepton to the rRNA fragment 1051-1109 are linked in a thermodynamic cycle such that the binding of either one facilitates the binding of the other.
- Thermodynamic cycle linking binding of thiostrepton (X) to the L11-binding domain (rRNA) of 23S rRNA. Formation of the ternary complex proceeds via either binary complex, rRNA•X or rRNA•L11. Thiostrepton has no affinity for free L11.
- Free energy profile for the binary and ternary complexes. The free energies ΔG°1, ΔG°2, and ΔG°exptl are calculated from observed values for the different association constants, Ka, using the equation ΔG° = -RT ln Ka. Macromolecular crowding and ion effects likely alter the binding affinities in the cell. However, as long as all the association constants are measured under identical conditions, ΔΔG will be the same in the cell and the test tube.
