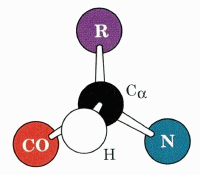
The "CORN" Acronym
You are using a web browser that is not fully supported by this website. Some features may not work as intended. For the best experience, please use one of the recommended browsers.

Opening image: Backbone trace of the B and E helices in myoglobin. The close approach of the two helices is possible because two invariant glycine residues (B6 and E8) are present at the crossing. Any side chain larger than a hydrogen atom pushes the helices apart, thereby disrupting the tertiary structure.
the rest of the protein.
spacefilling of Gly residues. The α carbons are shown in green.
spacefilling of the E helix. Use the mnemonic to imagine where the methyl group would be if GLY B6 (cyan helix) were replaced with alanine.
highlighting of the side chain of the glycines. The R group (a single hydrogen) is shown in magenta.
The heme is now shown along with the B and E helices. Toggle the following buttons to reveal the two invariant residues on the distal side of the heme: and .
Now let's examine the B helix. and are highly conserved in myoglobin and hemoglobin A and B chains. also forms non-covalent interactions with the heme.
We will explore binding of oxygen in the next section.