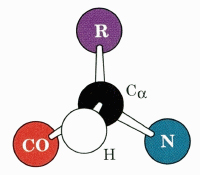
The "CORN" Acronym
You are using a web browser that is not fully supported by this website. Some features may not work as intended. For the best experience, please use one of the recommended browsers.

Cartoon representation of a very tight turn linking adjacent strands of an antiparallel β sheet. The structure reversing the direction of the polypeptide chain is a β turn, a third type of secondary structure. Nine residues are depicted.
spacefilling model without side chains. Note the tight packing of the backbone atoms in the turn (residues 104 to 107).
It's easy to identify the hydrogen bonds in the β sheet if we change colors to CPK colors. [Note: α carbons are dark gray.]
Residues 105 and 106 are not involved in the hydrogen-bonding pattern of the β sheet. The peptide H bond between 104 and 107 is the last H-bond in the sheet. However, 104 and 107 are included in the turn. Thus, the β turn is defined by four residues.
This view shows the 4 residues of the turn and 5 additional residues from the two β strands. Locate the glycine in the β turn.
the β turn without side chains (glycine's hydrogen is shown). Beta carbons are shown in green. Take a moment to appreciate the elegance of the turn.
Use the acronym to identify the α hydrogen that would be replaced by an R group if another amino acid were substituted Gly. Now you see why Gly is so important.
The tetrapeptide forming this particular β turn is Tyr-Glu-Gly-Val. This happens to be a Type II β turn; glycine is always found in the third position of Type II turns. Although a β turn consists of only four amino acids, many different types of β turns exist in proteins. The formation of the different types depends primarily on the tetrapeptide sequence.