Figure 1: Spacing of Side Chains in an α-Helix.
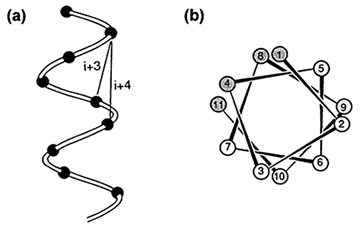
A.) Spacing of the Cα atoms of the main chain. Since there are 3.6 amino acid residues per turn, every 3rd or 4th Cα atom is on the same side of the helix.
B.) An amphipathic helix. The view is end-on looking from the amino- to carboxyl-terminal amino acid. This type of representation is referred to as a helical wheel. The shaded residues are strongly hydrophobic. In this example, the spacing between residues in the hydrophobic face follow the i + 3 and i + 4 pattern: i + 3 = 1 + 3 = 4; i + 4 = 4 + 4 = 8; and i + 3 = 8 + 3 = 11.