The "CORN" Acronym
You are using a web browser that is not fully supported by this website. Some features may not work as intended. For the best experience, please use one of the recommended browsers.

All four aromatic residues contain one methylene groups as a spacer. Aromatic rings are quite bulky and would prevent bends in the main chain if the methylene group were absent. Aromatic rings prefer to be in the interior of the protein, away from water.
is completely nonpolar; the aromatic ring is as nonpolar as benzene. Note that the other three aromatics have hydrogen bonding groups at the edge of the aromatic plane. These must be hydrogen bonded at all times. Hence , with its phenolic –OH group, and , with its indole –NH group, are often found near the surface of the protein. Most of the aromatic plane is "buried" but the polar edge is hydrogen bonded to water.
The side chain of is simply the methyl group.
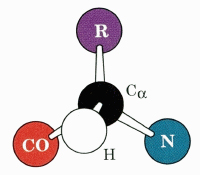
the α-carbon.
In 19 of the 20 amino acids used for the biosynthesis of proteins, the α-carbon atom is chiral, since four different groups are attached to it. The exception is glycine, which has two hydrogen atoms attached to the α-carbon. All 19 chiral amino acids are in the L configuration, where "L" signifies that the amino acid has the same configuration as L-glyceraldehyde.
Click to learn how to recognize the L configuration.
the methyl (R) group.
spacefilling model.
has an aromatic side chain.
the α-carbon.
the side chain.
All aromatic amino acids have a methylene group (β-carbon atom) between the bulky aromatic ring and the α-carbon atom.
the β-carbon atom dark green.
spacefilling model. The methylene group is very important. Without it the bulky aromatic ring would make the polypeptide chain too stiff for protein folding.
The phenolic –OH group of tyrosine is a very weak acid (pK = 11.1). The hydroxyl group can be phosphorylated by tyrosine protein kinases. At neutral pH, both tyrosine and tryptophan absorb UV light at 280 nm.
The indole side chain of tryptophan is the largest of the side chains. This amino acid also occurs least frequently, so proteins often have only one or two Trp residues. In indoles, two atoms of the benzene ring share a common carbon–carbon bond together with two carbon atoms of the pyrrole ring. A pyrrole is an aromatic five-membered ring with one nitrogen atom and two double bonds. Although it may seem that there are only four π-electrons in pyrrole, the pyrrole nitrogen atom has a of electrons. The pyrrole nitrogen atom is sp2 hybridized, and its unhybridized p orbital overlaps with the p orbitals of the carbon atoms to form a continuous ring. The lone pair on nitrogen occupies the p orbital and takes part in the π electron system.
The nitrogen atom in the indole ring of Trp is not basic. To form a bond to a proton the ring must use one of the electron pairs in the aromatic sextet. In the protonated indole, the nitrogen atom is bonded to four different atoms (two carbons and two hydrogens), requiring sp3 hybridization. Since this leaves no unhybridized p orbital, the protonated indole is nonaromatic.
The Trp side chain, being the largest of the side chains, is difficult to fit into protein structures. This has several consequences:
Histidine is an aromatic amino acid. All five atoms in the planar imidazole ring are sp2 hybridized. One of the nitrogen atoms (the one not bonded to a hydrogen) has its lone pair electrons in an that is not involved in the aromatic π-electron system; this lone pair is basic. The other nitrogen uses its third sp2 orbital to bond to hydrogen; its lone pair electrons occupy the and take part in the π-bonding system.
Although His is often classified with the basic amino acids (lysine and arginine), the imidazolium ion has a pK value between 6 and 7, depending on the microenvironment. Therefore, His is not fully ionized at pH 7, whereas the side chains of lysine and arginine bear a full positive charge at pH 7. His is frequently found in the active sites of enzymes, where it can donate or accept protons during catalysis.
The nitrogen atom that has the is an excellent nucleophile. The atoms bonded to this nitrogen atom are held back by the ring and offer relatively little steric hindrance to the sp2 lone pair. Thus, imidazole is one of the strongest nucleophiles that can exist at pH 7. His often participates in transfers of high-energy phosphoryl groups -- the intermediate phosphohistidine is a high-energy compound and can phosphorylate ADP to generate ATP.
Finally, imidazole can form hydrogen bonds with both hydrogen bond donors and acceptors. The imidazolium ion can form charged (strong) hydrogen bonds with hydrogen bond acceptors.