Of all the D-aldohexoses, β-D-glucose is the only one that can adopt a conformation with all its bulky groups in an equatorial position. With this advantage of stability, it may come as no surprise that β-D-glucose is the most widely occurring organic group in nature and the metabolic precursor of virtually all other biomolecules, including of course ribose-5-phosphate.
The notion of an "RNA world" was proposed in the 1960s as a way to explain the development of life on earth. If an RNA-only world preceded more complex forms of life, then it is essential that the process whereby the first nucleotides were made be considered. Various syntheses of the purine and pyrimidine bases from prebiotic molecules such as hydrogen cyanide and cyanamide have been described, perhaps the most notable being the synthesis of adenine from five molecules of HCN. However, no process has been have found for the prebiotic synthesis of nucleosides.
A bigger problem concerns the prebiotic origin of ribose. The condensation of formaldehyde into sugars was discovered in 1861, with glucose as a major product. Unfortunately, yields of ribose are very low -- and then only as the ribopyranose (6-membered ring) isomer, not ribofuranose (5-membered ring). Ribofuranose exists in cells, but only as ribose-5-phosphate and in ribonucleotides.
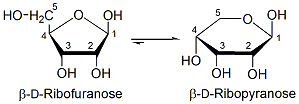
Albert Eschenmoser was the first to think outside the box and approach the problem by looking for prebiotically plausible alternatives to the ribofuranose-phosphate backbone. Since the transfer of genetic information does not depend the ribose-phosphate backbone, it is logical to propose that a simpler replication system would have appeared before the RNA molecule. In the 1990s Eschenmoser's group set out to explore systematically the properties of polymers in which the 5′,3′-linked ribose-phosphate backbone of RNA was replaced by some other sugar-phosphate backbone. In the first experiments Eschenmoser's group explored the properties of pyranose-RNA (p-RNA) in which ribofuranose is replaced by ribopyranose. p-RNA forms more stable double helices with Watson-Crick pairings than does RNA with ribofuranose. p-RNA could therefore be a good candidate as a precursor genetic systems.
Recently Eschenmoser's group synthesized polymers in which the ribose group was replaced by 2′,3′-dideoxyglucose, whose prebiotic synthesis seems easier. The only chemical difference between DNA and homo-DNA is the insertion of a CH2 group into the sugar ring; everything else is unchanged. Both pyranose model systems, p-RNA and homo-DNA, form stable base-paired double helices. A complete understanding of the properties of the pyranose family of nucleic acids was not possible until the crystal structure of homo-DNA was published in 2006 (PDB code 2H9S).
Further Reading
-
Stano, P. and P. L. Luisi (2007). Basic Questions About the Origins of Life: Proceedings of the Erice International School of Complexity (Fourth Course) Orig Life Evol Biosph 37:303-307.
This is a list of nine questions prepared by the authors, who were organizers of an international meeting held at Erice, Italy in 2006. The meeting focused on the origin of life, and the organizers asked those planning to attend the meeting to submit answers to one or more of the questions in advance of the meeting. Thirty three of the short answers have been published in a special issue of the journal, "Origins of Life and Evolution of Biospheres." See below for links to several articles of interest. - Avetisov, V. (2007). Question 4: Short Remarks About the Origin of Homochirality Orig Life Evol Biosph 37:367-370
- Lucrezia, D. F. Anella, and C. Chiarabelli (2007). Question 5: On the Chemical Reality of the RNA World Orig Life Evol Biosph 37:379-385.
- Monnard, P.-A. (2007). Question 5: Does the RNA-world still retain its appeal after 40 years of research? Orig Life Evol Biosph 37:387-390